SAN DIEGO, Sept. 22, 2025 /PRNewswire/ -- Neurocrine Biosciences, Inc. (NASDAQ:NBIX) today announced the presentation of new data from the Phase 2 SAVITRI™ study, which showed statistically significant and clinically meaningful improvement in depression severity at Day 28 and Day 56 with once-daily oral administration of 1 mg osavampator (NBI-1065845). In recognition of the data presentation, Neurocrine was honored with the 2025 Poster Award at the 38th annual Psych Congress in San Diego.
"Major depressive disorder is one of the most common mental health conditions, and up to half of patients do not get sufficient relief from their current antidepressant regimen," said Sanjay Keswani, M.D., Chief Medical Officer, Neurocrine Biosciences. "We are encouraged by these results that show osavampator may help address this unmet need by modulating AMPA receptor activity."
In this dose-finding study, 183 adults 18 to 65 years of age were randomized in a 2:1:1 ratio to receive placebo, osavampator 1 mg or osavampator 3 mg once daily for eight weeks. Osavampator is an investigational potential first-in-class selective positive allosteric modulator of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA-PAM) in development as a potential adjunctive treatment for adults with major depressive disorder (MDD) who have an inadequate response to oral antidepressant treatment.
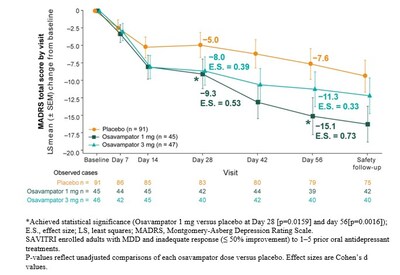
The study met its primary efficacy endpoint, showing a significant reduction in depression severity from baseline to Day 28 compared to placebo, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) total score. Secondary efficacy endpoints included the change in MADRS total score from baseline to Day 56.
Additional Secondary Efficacy Endpoints
Treatment Response (≥50% reduction in MADRS total score from baseline):
- Day 56: osavampator 1 mg maintained statistical significance; 3 mg showed favorable but nominal difference
Remission (MADRS score ≤10):
- Day 56: osavampator 1 mg achieved statistically significant improvement versus placebo; 3 mg showed numerical improvement without statistical significance
Osavampator was generally well tolerated at both doses, with no serious adverse events or adverse events of special interest reported. The most commonly reported treatment-emergent adverse events (TEAEs) occurring in 5% or more of participants receiving osavampator were headache and nasopharyngitis, which were similar in occurrence to placebo.
TEAE Occurring in ≥5% of Participants in Any Treatment Group
Preferred Term
| Placebo
(N=91)
n (%)
| osavampator 1 mg
(N=45)
n (%)
| osavampator 3 mg
(N=47)
n (%)
|
Any TEAE
| 39 (42.9)
| 25 (55.6)
| 21 (44.7)
|
Headache
| 8 (8.8)
| 5 (11.1)
| 2 (4.3)
|
Nasopharyngitis
| 5 (5.5)
| 2 (4.4)
| 3 (6.4)
|
Insomnia
| 2 (2.2)
| 1 (2.2)
| 3 (6.4)
|
Somnolence
| 3 (3.3)
| 3 (6.7)
| 0
|
Nausea
| 5 (5.5)
| 1 (2.2)
| 0
|
Dizziness
| 5 (5.5)
| 0
| 0
|
Data shown are the number of subjects reporting at least one occurrence of the event within each treatment group.
Results of an exploratory post hoc exposure-response analysis substantiate the continued evaluation of the 1 mg once-daily dosing in the phase 3 studies in MDD.
Poster presentations at Psych Congress 2025 include:
- Osavampator (NBI-1065845/TAK-653) Demonstrates Statistically Significant and Clinically Meaningful Improvements in Depression Severity and is Well Tolerated in Adults with Major Depressive Disorder: Phase 2 SAVITRI Results
- Osavampator: A Selective Positive Allosteric Modulator of the AMPA Receptor (AMPA-PAM) in Development for the Treatment of Major Depressive Disorder
- The Functional Impact of Major Depressive Disorder on Patients' Daily Lives: A Qualitative Investigation of Patient and Clinician Perspectives
- Once-Daily Valbenazine Improves the Impacts and Symptoms of Tardive Dyskinesia Regardless of Psychiatric Diagnosis: Results from the Phase 4 KINECT-PROTM Study
- Once-Daily Valbenazine Improves the Impacts of Tardive Dyskinesia (TD) in Patients Who Met a Threshold for TD Remission: Post Hoc Analyses of Patient-Reported Outcomes from KINECT-PRO
- Valbenazine Improves Physical, Social, and Emotional Impacts on the Tardive Dyskinesia Impact Scale (TDIS™): Post Hoc Analyses of KINECT-PRO Data
- Estimation of the Minimal Clinically Important Difference and Longitudinal Change in the Tardive Dyskinesia Impact Scale, a Validated, Tardive Dyskinesia-Specific, Patient-Reported Outcome Measure
- Physical, Mental, and Socioemotional Functional Improvement Following Valbenazine Treatment for TD: a Case Series
- Once-Daily Valbenazine for the Treatment of Tardive Dyskinesia in Elderly Adults and Other Special Populations
- Valbenazine Improves Tardive Dyskinesia in Patients Regardless of Ethnicity or Race: Post Hoc Analyses of Long-Term Data from the KINECT® 4 Study
- A Qualitative, Interview-based Study of Patient, Caregiver, and Prescriber Rankings of Functional Outcome Improvements in Schizophrenia
About Osavampator and the Phase 2 SAVITRI™ Study
Osavampator (NBI-1065845) is an investigational selective positive allosteric modulator of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA-PAM) in development as a potential adjunctive treatment for adults with major depressive disorder (MDD) who have an inadequate response to oral antidepressant treatment. Neurocrine received an exclusive license to osavampator from Takeda Pharmaceutical Company Limited for all indications in all territories worldwide except Japan.
The Phase 2 SAVITRI study was a double-blind, placebo-controlled study designed to assess the efficacy and safety of investigational osavampator in adult subjects with MDD. The study enrolled 183 adults with a primary diagnosis of MDD and who had an inadequate response to current antidepressant treatment.
The Phase 3 Registrational Program
Following the announcement of positive top-line data from the Phase 2 SAVITRI study, Neurocrine initiated a Phase 3 registrational program of osavampator in January 2025, which includes five studies that are currently active and enrolling. For more information about the Phase 3 osavampator studies, please visit: ClinicalTrials.gov.
About Major Depressive Disorder
Major depressive disorder is a serious disorder characterized by a persistently depressed mood, loss of interest, poor concentration, and decreased energy, among other symptoms. According to the World Health Organization, MDD is one of the leading causes of disability, is a serious condition that presents an increased risk of suicide and self-harm and is associated with increased all-cause mortality. More than 21 million people in the U.S. live with MDD, and about half of patients do not get enough relief from their first antidepressant.
About Neurocrine Biosciences, Inc.
Neurocrine Biosciences is a leading neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, chorea associated with Huntington's disease, classic congenital adrenal hyperplasia, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders because you deserve brave science. For more information, visit neurocrine.com, and follow the company on LinkedIn, X and Facebook. (*in collaboration with AbbVie)
The NEUROCRINE BIOSCIENCES Logo, NEUROCRINE, KINECT and YOU DESERVE BRAVE SCIENCE are registered trademarks of Neurocrine Biosciences, Inc. SAVITRI, TDIS and KINECT-PRO are trademarks of Neurocrine Biosciences, Inc.
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements regarding the clinical results from, and our future development plans with respect to, osavampator (NBI-1065845), the therapeutic potential and clinical benefits or safety profile of osavampator, and the potential benefits to be derived from certain of our products. Factors that could cause actual results to differ materially from those stated or implied in the forward-looking statements include, but are not limited to, the following: data that we report may change following a more comprehensive review of the data related to the clinical study and such data may not accurately reflect the complete results of the clinical study; risks that clinical development activities may not be initiated or completed on time or at all, or may be delayed for regulatory, manufacturing or other reasons, may not be successful or replicate previous clinical trial results, may fail to demonstrate that our product candidates are safe and effective, or may not be predictive of real-world results or of results in subsequent clinical trials; risks that regulatory submissions for our product candidates may not occur or be submitted in a timely manner; our future financial and operating performance; risks associated with our dependence on third parties for development, manufacturing and commercialization activities for our products and product candidates and our ability to manage these third parties; risks that the FDA or other regulatory authorities may make adverse decisions regarding our products or product candidates; risks that the potential benefits of the agreements with our collaboration partners may never be realized; risks that our products and/or our product candidates may be precluded from commercialization by the proprietary or regulatory rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; risks associated with U.S. federal or state legislative or regulatory and/or policy efforts which may result in, among other things, an adverse impact on our revenues or potential revenue; risks associated with potential generic entrants for our products; and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission, including without limitation the Company's quarterly report on Form 10-Q for the quarter ended June 30, 2025. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof other than required by law.
© 2025 Neurocrine Biosciences, Inc. All Rights Reserved
 View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-presents-positive-new-data-from-phase-2-study-of-osavampator-in-adults-with-major-depressive-disorder-302562476.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurocrine-biosciences-presents-positive-new-data-from-phase-2-study-of-osavampator-in-adults-with-major-depressive-disorder-302562476.html
SOURCE Neurocrine Biosciences, Inc.